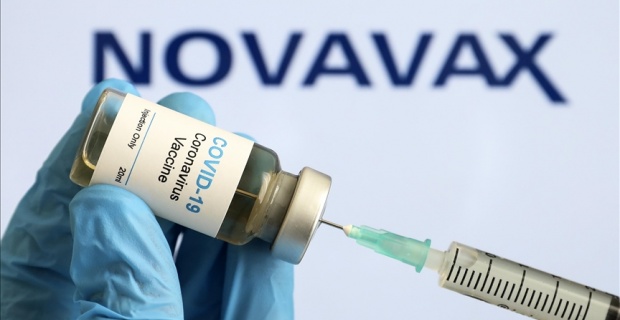
The British government approved a fifth coronavirus vaccine on Thursday for those aged 18 and older. The Nuvaxovid vaccine, produced by US company Novavax, is the first protein-based coronavirus vaccine and provides a 90% protection rate against the virus and various mutations, said the Medicines and Healthcare products Regulatory Agency (MHRA).
“Our approval of Nuvaxovid today follows a rigorous review of the safety, quality and effectiveness of this vaccine, and expert advice from the government’s independent scientific advisory body, the Commission on Human Medicines,” said June Raine, chief executive of the MHRA.
The Independent Commission on Human Medicines also confirmed the benefits of the Nuvaxovid vaccine, and advised the population to accept it should they be offered the jab.
“Nuvaxovid is distinct from other COVID-19 vaccines currently in use in the UK as it uses recombinant protein-based technology which has been used for many years in the development of vaccines to prevent other illnesses, for example Hepatitis B,” said Sir Munir Pirmohamed of the Commission.
Health Secretary Sajid Javid described the development as "testament" to the UK's "world-leading science and research expertise."
The jab, however, will not be immediately available as a review will have to be undertaken by the Joint Committee on Vaccination and Immunisation and upon consideration, the vaccine will be rolled out to hospitals, clinics and pharmacies across the country.
The UK’s Vaccine Taskforce placed an order for 60 million doses of the Nuvaxovid vaccine in 2020 which were expected in the second half of 2021.
The delivery, however, was beset by delays because of manufacturing problems and US regulatory processes.
The COVID-19 vaccines currently approved for use in the UK are Moderna, Oxford/AstraZeneca, Pfizer/BioNTech and Johnson & Johnson’s Janssen.


 After Nesil Caliskan a by-election will be held in Jubilee ward in Enfield
After Nesil Caliskan a by-election will be held in Jubilee ward in Enfield Publishing the analysis, Labour’s Cllr Ergin Erbil said Everybody in Enfield deserves basic rights
Publishing the analysis, Labour’s Cllr Ergin Erbil said Everybody in Enfield deserves basic rights Gaza-Israel conflict Statement from Cllr Ergin Erbil, Leader of Enfield Council
Gaza-Israel conflict Statement from Cllr Ergin Erbil, Leader of Enfield Council Cllr Ergin Erbil was elected as the new Leader of Enfield Council
Cllr Ergin Erbil was elected as the new Leader of Enfield Council The European Union called on Turkey to uphold democratic values
The European Union called on Turkey to uphold democratic values Turkish citizens in London said Rights, Law, Justice
Turkish citizens in London said Rights, Law, Justice The Council of Turkish Cypriot Associations Geneva response letter
The Council of Turkish Cypriot Associations Geneva response letter Sustainable Development and ESG, Will This Become the Course for Turkic World
Sustainable Development and ESG, Will This Become the Course for Turkic World The 'Prince of Paris' has impressed in his first EuroLeague season
The 'Prince of Paris' has impressed in his first EuroLeague season Saran Media And Euroleague Basketball Extend Media Rights Partnership for Four More Years
Saran Media And Euroleague Basketball Extend Media Rights Partnership for Four More Years Will Rangers be Jose Mourinho’s next victim?
Will Rangers be Jose Mourinho’s next victim? Jose Mourinho's Fenerbahce face Rangers on Thursday
Jose Mourinho's Fenerbahce face Rangers on Thursday Barclays has become the biggest UK lender so far to cut mortgage rates
Barclays has become the biggest UK lender so far to cut mortgage rates THE SPRING STATEMENT EXPLAINED, UK ECONOMIC OUTLOOK AND GROWTH FORECASTS
THE SPRING STATEMENT EXPLAINED, UK ECONOMIC OUTLOOK AND GROWTH FORECASTS Launch of Made in Enfield gift shop to celebrate local artists and designers
Launch of Made in Enfield gift shop to celebrate local artists and designers Trial used smart Wi-Fi sensors for live building occupancy data to optimise
Trial used smart Wi-Fi sensors for live building occupancy data to optimise